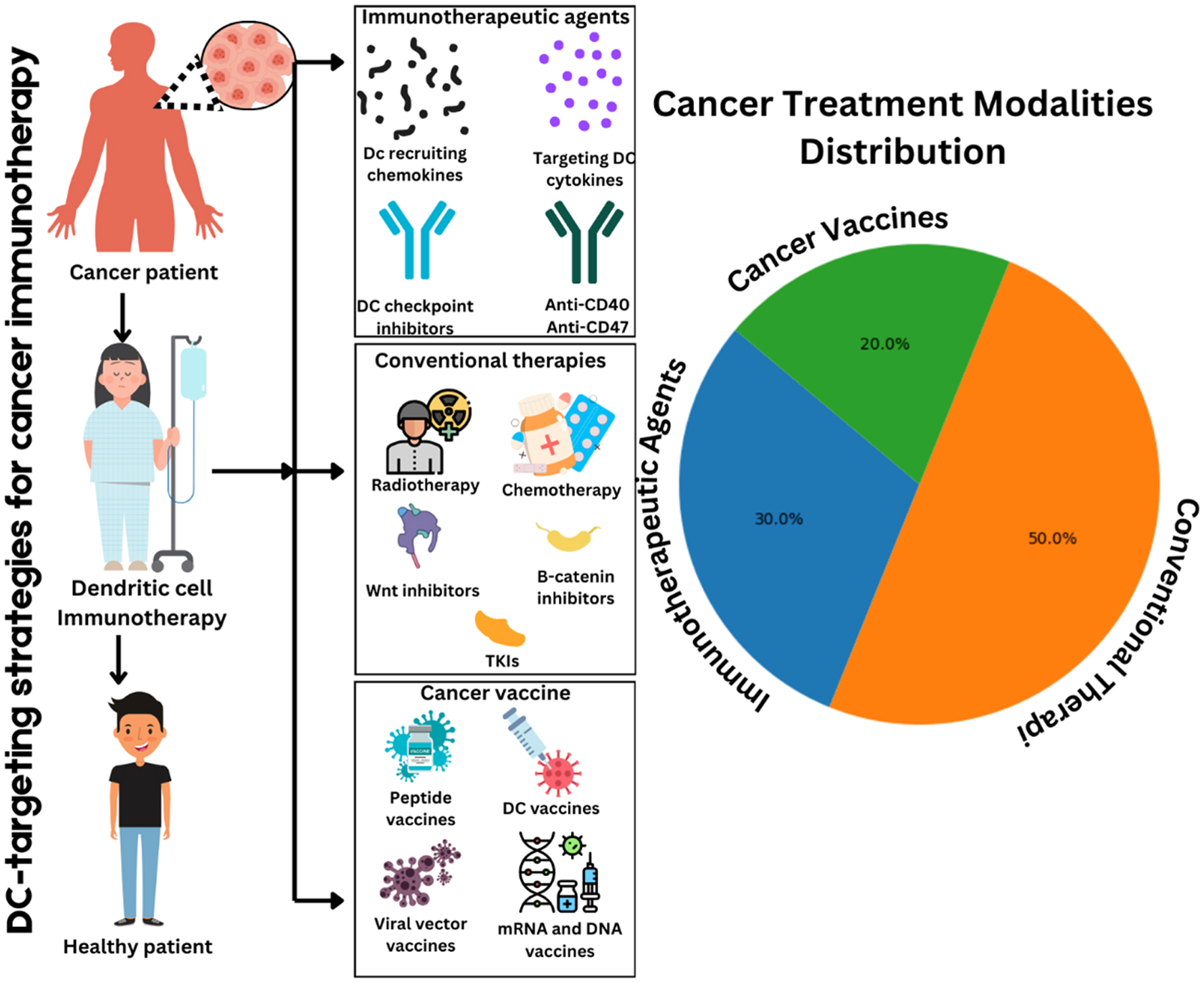
Gastric cancer (GC) remains a significant global health challenge, ranking as the fifth most common cancer and the third leading cause of cancer-related deaths worldwide. Despite declining incidence rates in certain regions, such as the United States, mortality remains high, particularly in Eastern Asia. In this review, we explore innovative immunotherapeutic strategies for GC, including immune checkpoint inhibitors, CAR-T cell therapy, and therapeutic vaccines. The role of the tumor microenvironment (TME), immune cell interactions, tumor acidity, and microbial flora in shaping therapeutic responses is discussed. Emerging epidemiological trends, such as the rise of cardia gastric cancer and shifts in traditional risk factor profiles, underscore the need for targeted prevention strategies. While immunotherapy offers promising opportunities, challenges like therapeutic resistance and personalization of treatment persist. We aimed to highlight advancements in GC immunotherapy to promote research and improve patient outcomes.
Citation: Mujibullah Sheikh, Pranita S. Jirvankar. Innovative immunotherapeutic strategies for gastric cancer: A comprehensive review[J]. AIMS Allergy and Immunology, 2025, 9(1): 27-55. doi: 10.3934/Allergy.2025003
Gastric cancer (GC) remains a significant global health challenge, ranking as the fifth most common cancer and the third leading cause of cancer-related deaths worldwide. Despite declining incidence rates in certain regions, such as the United States, mortality remains high, particularly in Eastern Asia. In this review, we explore innovative immunotherapeutic strategies for GC, including immune checkpoint inhibitors, CAR-T cell therapy, and therapeutic vaccines. The role of the tumor microenvironment (TME), immune cell interactions, tumor acidity, and microbial flora in shaping therapeutic responses is discussed. Emerging epidemiological trends, such as the rise of cardia gastric cancer and shifts in traditional risk factor profiles, underscore the need for targeted prevention strategies. While immunotherapy offers promising opportunities, challenges like therapeutic resistance and personalization of treatment persist. We aimed to highlight advancements in GC immunotherapy to promote research and improve patient outcomes.

Gastric cancer
Gastrointestinal stromal tumors
Mortality-to-incidence ratio
Carcinoma-associated fibroblasts
Immune checkpoint inhibitors
Tumor microenvironment
Tumor-associated macrophages
Myeloid-derived suppressor cells
Colony-stimulating factor 1 receptor
programmed cell death protein 1
programmed cell death ligand 1
Triple-negative breast cancer
Hepatocellular carcinoma
Renal cell carcinoma
Endometrial cancer

| [1] | Mukkamalla SKR, Recio-Boiles A, Babiker HM (2024) Gastric Cancer. Florida: StatPearls Publishing. Available from: http://www.ncbi.nlm.nih.gov/books/NBK459142/ |
| [2] |
De Martel C, Forman D, Plummer M (2013) Gastric cancer. Gastroenterol Clin North Am 42: 219-240. https://doi.org/10.1016/j.gtc.2013.01.003 
|
| [3] |
Karimi P, Islami F, Anandasabapathy S, et al. (2014) Gastric cancer: Descriptive epidemiology, risk factors, screening, and prevention. Cancer Epidemiol Biomarkers Prev 23: 700-713. https://doi.org/10.1158/1055-9965.EPI-13-1057 
|
| [4] |
Rawla P, Barsouk A (2019) Epidemiology of gastric cancer: Global trends, risk factors and prevention. Gastroenterol Rev 14: 26-38. https://doi.org/10.5114/pg.2018.80001 
|
| [5] |
Sitarz R, Skierucha M, Mielko J, et al. (2018) Gastric cancer: Epidemiology, prevention, classification, and treatment. Cancer Manage Res 10: 239-248. https://doi.org/10.2147/CMAR.S149619 
|
| [6] |
Morgan E, Arnold M, Camargo MC, et al. (2022) The current and future incidence and mortality of gastric cancer in 185 countries, 2020–40: A population-based modelling study. EClinicalMedicine 47: 101404. https://doi.org/10.1016/j.eclinm.2022.101404 
|
| [7] |
Wong MCS, Huang J, Chan PSF, et al. (2021) Global Incidence and mortality of gastric cancer, 1980–2018. JAMA Network Open 4: e2118457. https://doi.org/10.1001/jamanetworkopen.2021.18457 
|
| [8] | Hua MJ Staging efficacy: Cancer treatment in contemporary China (2020). https://doi.org/10.6082/uchicago.2753 |
| [9] |
Lin JL, Lin JX, Lin GT, et al. (2024) Global incidence and mortality trends of gastric cancer and predicted mortality of gastric cancer by 2035. BMC Public Health 24: 1763. https://doi.org/10.1186/s12889-024-19104-6 
|
| [10] |
Miller KD, Nogueira L, Mariotto AB, et al. (2019) Cancer treatment and survivorship statistics, 2019. CA A Cancer J Clinicians 69: 363-385. https://doi.org/10.3322/caac.21565 
|
| [11] |
Miller KD, Nogueira L, Devasia T, et al. (2022) Cancer treatment and survivorship statistics, 2022. Ca-Cancer J Clin 72: 409-436. https://doi.org/10.3322/caac.21731 
|
| [12] |
Malik A, Ali F, Malik MI, et al. (2024) The risk of infection-caused mortality in gastric adenocarcinoma: A population-based study. Gastroenterol Res 17: 133-145. https://doi.org/10.14740/gr.v17i3.1715 
|
| [13] |
Assumpção PP, Barra WF, Ishak G, et al. (2020) The diffuse-type gastric cancer epidemiology enigma. BMC Gastroenterol 20: 223. https://doi.org/10.1186/s12876-020-01354-4 
|
| [14] |
Iyer P, Moslim M, Farma JM, et al. (2020) Diffuse gastric cancer: Histologic, molecular, and genetic basis of disease. Transl Gastroenterol Hepatol 5: 52. https://doi.org/10.21037/tgh.2020.01.02 
|
| [15] |
Mabula JB, Mchembe MD, Koy M, et al. (2012) Gastric cancer at a university teaching hospital in northwestern Tanzania: A retrospective review of 232 cases. World J Surg Oncol 10: 257. https://doi.org/10.1186/1477-7819-10-257 
|
| [16] |
Iwu CD, Iwu-Jaja CJ (2023) Gastric cancer epidemiology: Current trend and future direction. Hygiene 3: 256-268. https://doi.org/10.3390/hygiene3030019 
|
| [17] |
Rugge M, Fassan M, Graham DY (2015) Epidemiology of gastric cancer. Gastric Cancer . Cham: Springer International Publishing 23-34. https://doi.org/10.1007/978-3-319-15826-6_2 
|
| [18] |
Ferro A, Peleteiro B, Malvezzi M, et al. (2014) Worldwide trends in gastric cancer mortality (1980–2011), with predictions to 2015, and incidence by subtype. Eur J Cancer 50: 1330-1344. https://doi.org/10.1016/j.ejca.2014.01.029 
|
| [19] |
Machlowska J, Baj J, Sitarz M, et al. (2020) Gastric cancer: Epidemiology, risk factors, classification, genomic characteristics and treatment strategies. Int J Mol Sci 21: 4012. https://doi.org/10.3390/ijms21114012 
|
| [20] |
Hu Y, Huang C, Sun Y, et al. (2016) Morbidity and mortality of laparoscopic versus open D2 distal gastrectomy for advanced gastric cancer: A randomized controlled trial. J Clin Oncol 34: 1350-1357. https://doi.org/10.1200/JCO.2015.63.7215 
|
| [21] |
Naran K, Nundalall T, Chetty S, et al. (2018) Principles of immunotherapy: Implications for treatment strategies in cancer and infectious diseases. Front Microbiol 9: 3158. https://doi.org/10.3389/fmicb.2018.03158 
|
| [22] |
Hu H, Chen Y, Tan S, et al. (2022) The research progress of antiangiogenic therapy, immune therapy and tumor microenvironment. Front Immunol 13: 802846. https://doi.org/10.3389/fimmu.2022.802846 
|
| [23] |
Bilotta MT, Antignani A, Fitzgerald DJ (2022) Managing the TME to improve the efficacy of cancer therapy. Front Immunol 13: 954992. https://doi.org/10.3389/fimmu.2022.954992 
|
| [24] |
Samstein RM, Lee CH, Shoushtari AN, et al. (2019) Tumor mutational load predicts survival after immunotherapy across multiple cancer types. Nat Genet 51: 202-206. https://doi.org/10.1038/s41588-018-0312-8 
|
| [25] |
Ru B, Wong CN, Tong Y, et al. (2019) TISIDB: An integrated repository portal for tumor–immune system interactions. Bioinformatics 35: 4200-4202. https://doi.org/10.1093/bioinformatics/btz210 
|
| [26] |
Bagchi S, Yuan R, Engleman EG (2021) Immune checkpoint inhibitors for the treatment of cancer: Clinical impact and mechanisms of response and resistance. Annu Rev Pathol Mech Dis 16: 223-249. https://doi.org/10.1146/annurev-pathol-042020-042741 
|
| [27] |
Labani-Motlagh A, Ashja-Mahdavi M, Loskog A (2020) The tumor microenvironment: A milieu hindering and obstructing antitumor immune responses. Front Immunol 11: 940. https://doi.org/10.3389/fimmu.2020.00940 
|
| [28] |
Zhu Y, Knolhoff BL, Meyer MA, et al. (2014) CSF1/CSF1R blockade reprograms tumor-infiltrating macrophages and improves response to T-cell checkpoint immunotherapy in pancreatic cancer models. Cancer Res 74: 5057-5069. https://doi.org/10.1158/0008-5472.CAN-13-3723 
|
| [29] |
Henke E, Nandigama R, Ergün S (2020) Extracellular matrix in the tumor microenvironment and its impact on cancer therapy. Front Mol Biosci 6: 160. https://doi.org/10.3389/fmolb.2019.00160 
|
| [30] |
Mager LF, Burkhard R, Pett N, et al. (2020) Microbiome-derived inosine modulates response to checkpoint inhibitor immunotherapy. Science 369: 1481-1489. https://doi.org/10.1126/science.abc3421 
|
| [31] |
Kon E, Benhar I (2019) Immune checkpoint inhibitor combinations: Current efforts and important aspects for success. Drug Resist Updates 45: 13-29. https://doi.org/10.1016/j.drup.2019.07.004 
|
| [32] |
Sobhani N, Tardiel-Cyril DR, Davtyan A, et al. (2021) CTLA-4 in regulatory T cells for cancer immunotherapy. Cancers 13: 1440. https://doi.org/10.3390/cancers13061440 
|
| [33] |
Tang Q, Chen Y, Li X, et al. (2022) The role of PD-1/PD-L1 and application of immune-checkpoint inhibitors in human cancers. Front Immunol 13: 964442. https://doi.org/10.3389/fimmu.2022.964442 
|
| [34] | Colligan SH Mitigating myeloid-driven pathways of immune suppression to enhance cancer immunotherapy efficacy (2022). |
| [35] |
Guzik K, Tomala M, Muszak D, et al. (2019) Development of the inhibitors that target the PD-1/PD-L1 interaction—a brief look at progress on small molecules, peptides and macrocycles. Molecules 24: 2071. https://doi.org/10.3390/molecules24112071 
|
| [36] |
Graziani G, Lisi L, Tentori L, et al. (2022) Monoclonal antibodies to CTLA-4 with focus on ipilimumab. Interaction of Immune and Cancer Cells . Cham: Springer International Publishing 295-350. https://doi.org/10.1007/978-3-030-91311-3_10 
|
| [37] |
Lipson EJ, Drake CG (2011) Ipilimumab: An anti-CTLA-4 antibody for metastatic melanoma. Clin Cancer Res 17: 6958-6962. https://doi.org/10.1158/1078-0432.CCR-11-1595 
|
| [38] |
Rajan A, Kim C, Heery CR, et al. (2016) Nivolumab, anti-programmed death-1 (PD-1) monoclonal antibody immunotherapy: Role in advanced cancers. Hum Vaccines Immunother 12: 2219-2231. https://doi.org/10.1080/21645515.2016.1175694 
|
| [39] |
Massard C, Gordon MS, Sharma S, et al. (2016) Safety and efficacy of durvalumab (MEDI4736), an anti–programmed cell death Ligand-1 immune checkpoint inhibitor, in patients with advanced urothelial bladder cancer. J Clin Oncol 34: 3119-3125. https://doi.org/10.1200/JCO.2016.67.9761 
|
| [40] |
Peters S, Kerr KM, Stahel R (2018) PD-1 blockade in advanced NSCLC: A focus on pembrolizumab. Cancer Treat Rev 62: 39-49. https://doi.org/10.1016/j.ctrv.2017.10.002 
|
| [41] |
Inman BA, Longo TA, Ramalingam S, et al. (2017) Atezolizumab: A PD-L1–blocking antibody for bladder cancer. Clin Cancer Res 23: 1886-1890. https://doi.org/10.1158/1078-0432.CCR-16-1417 
|
| [42] |
Teets A, Pham L, Tran EL, et al. (2018) Avelumab: A novel anti-PD-L1 agent in the treatment of merkel cell carcinoma and urothelial cell carcinoma. Crit Rev Immunol 38: 159-206. https://doi.org/10.1615/CritRevImmunol.2018025204 
|
| [43] | Rath B, Plangger A, Hamilton G (2020) Non-small cell lung cancer-small cell lung cancer transformation as mechanism of resistance to tyrosine kinase inhibitors in lung cancer. Cancer Drug Res 3: 171. https://doi.org/10.20517/cdr.2019.85 |
| [44] |
Villani A, Ocampo-Garza SS, Potestio L, et al. (2022) Cemiplimab for the treatment of advanced cutaneous squamous cell carcinoma. Expert Opin Drug Saf 21: 21-29. https://doi.org/10.1080/14740338.2022.1993819 
|
| [45] |
Mirza MR, Chase DM, Slomovitz BM, et al. (2023) Dostarlimab for primary advanced or recurrent endometrial cancer. N Engl J Me 388: 2145-2158. https://doi.org/10.1056/NEJMoa2216334 
|
| [46] |
Patel TH, Brewer JR, Fan J, et al. (2024) FDA approval summary: Tremelimumab in combination with durvalumab for the treatment of patients with unresectable hepatocellular carcinoma. Clin Cancer Res 30: 269-273. https://doi.org/10.1158/1078-0432.CCR-23-2124 
|
| [47] | Burtness B Toripalimab yields ‘Striking’ PFS improvement in nasopharyngeal carcinoma (2024). Available from: https://www.cancernetwork.com/view/toripalimab-yields-striking-pfs-improvement-in-nasopharyngeal-carcinoma |
| [48] |
Rugo HS, Kabos P, Beck JT, et al. (2022) Abemaciclib in combination with pembrolizumab for HR+, HER2− metastatic breast cancer: Phase 1b study. npj Breast Cancer 8: 1-8. https://doi.org/10.1038/s41523-022-00482-2 
|
| [49] |
Deng Y, Huang M, Deng R, et al. (2024) Immune checkpoint inhibitor-related adrenal hypofunction and Psoriasisby induced by tislelizumab: A case report and review of literature. Medicine 103: e37562. https://doi.org/10.1097/MD.0000000000037562 
|
| [50] |
Reuss JE, Gosa L, Liu SV (2021) Antibody drug conjugates in lung cancer: State of the current therapeutic landscape and future developments. Clin Lung Cancer 22: 483-499. https://doi.org/10.1016/j.cllc.2021.07.011 
|
| [51] |
Brahmer JR, Tykodi SS, Chow LQM, et al. (2012) Safety and activity of anti–PD-L1 antibody in patients with advanced cancer. N Engl J Med 366: 2455-2465. https://doi.org/10.1056/NEJMoa1200694 
|
| [52] |
Dermani FK, Samadi P, Rahmani G, et al. (2019) PD-1/PD-L1 immune checkpoint: Potential target for cancer therapy. J Cell Physiol 234: 1313-1325. https://doi.org/10.1002/jcp.27172 
|
| [53] |
Lai X, Friedman A (2017) Combination therapy for melanoma with BRAF/MEK inhibitor and immune checkpoint inhibitor: A mathematical model. BMC Syst Biol 11: 70. https://doi.org/10.1186/s12918-017-0446-9 
|
| [54] |
Zhang Q, Shao B, Tong Z, et al. (2022) A phase Ib study of camrelizumab in combination with apatinib and fuzuloparib in patients with recurrent or metastatic triple-negative breast cancer. BMC Med 20: 321. https://doi.org/10.1186/s12916-022-02527-6 
|
| [55] |
Dazio G, Epistolio S, Frattini M, et al. (2022) Recent and future strategies to overcome resistance to targeted therapies and immunotherapies in metastatic colorectal cancer. J Clin Med 11: 7523. https://doi.org/10.3390/jcm11247523 
|
| [56] |
Liu Y, Zhang X, Wang G, et al. (2021) Triple combination therapy with PD-1/PD-L1, BRAF, and MEK inhibitor for stage III–IV melanoma: A systematic review and meta-analysis. Front Oncol 11: 693655. https://doi.org/10.3389/fonc.2021.693655 
|
| [57] |
Stein MK, Oluoha O, Patel K, et al. (2021) Precision medicine in oncology: A review of multi-tumor actionable molecular targets with an emphasis on non-small cell lung cancer. J Pers Med 11: 518. https://doi.org/10.3390/jpm11060518 
|
| [58] |
Musacchio L, Cicala CM, Camarda F, et al. (2022) Combining PARP inhibition and immune checkpoint blockade in ovarian cancer patients: A new perspective on the horizon?. ESMO Open 7: 100536. https://doi.org/10.1016/j.esmoop.2022.100536 
|
| [59] |
Rizvi H, Sanchez-Vega F, La K, et al. (2018) Molecular determinants of response to anti–programmed cell death (PD)-1 and anti–programmed death-Ligand 1 (PD-L1) blockade in patients with non–small-cell lung cancer profiled with targeted next-generation sequencing. J Clin Oncol 36: 633-641. https://doi.org/10.1200/JCO.2017.75.3384 
|
| [60] |
Gettinger S, Choi J, Hastings K, et al. (2017) Impaired HLA class I antigen processing and presentation as a mechanism of acquired resistance to immune checkpoint inhibitors in lung cancer. Cancer Disco 7: 1420-1435. https://doi.org/10.1158/2159-8290.CD-17-0593 
|
| [61] |
Sibaud V (2018) Dermatologic Reactions to immune checkpoint inhibitors: Skin toxicities and immunotherapy. Am J Clin Dermatol 19: 345-361. https://doi.org/10.1007/s40257-017-0336-3 
|
| [62] |
Ribas A, Wolchok JD (2018) Cancer immunotherapy using checkpoint blockade. Science 359: 1350-1355. https://doi.org/10.1126/science.aar4060 
|
| [63] |
Vétizou M, Pitt JM, Daillère R, et al. (2015) Anticancer immunotherapy by CTLA-4 blockade relies on the gut microbiota. Science 350: 1079-1084. https://doi.org/10.1126/science.aad1329 
|
| [64] |
Van Allen EM, Miao D, Schilling B, et al. (2015) Genomic correlates of response to CTLA-4 blockade in metastatic melanoma. Science 350: 207-211. https://doi.org/10.1126/science.aad0095 
|
| [65] |
Wang DY, Salem JE, Cohen JV, et al. (2018) Fatal toxic effects associated with immune checkpoint inhibitors: A systematic review and meta-analysis. JAMA Oncol 4: 1721. https://doi.org/10.1001/jamaoncol.2018.3923 
|
| [66] |
Rowshanravan B, Halliday N, Sansom DM (2018) CTLA-4: A moving target in immunotherapy. Blood 131: 58-67. https://doi.org/10.1182/blood-2017-06-741033 
|
| [67] |
Qin S, Xu L, Yi M, et al. (2019) Novel immune checkpoint targets: Moving beyond PD-1 and CTLA-4. Mol Cancer 18: 155. https://doi.org/10.1186/s12943-019-1091-2 
|
| [68] |
Rizvi NA, Hellmann MD, Snyder A, et al. (2015) Mutational landscape determines sensitivity to PD-1 blockade in non–small cell lung cancer. Science 348: 124-128. https://doi.org/10.1126/science.aaa1348 
|
| [69] |
Routy B, Le Chatelier E, Derosa L, et al. (2018) Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science 359: 91-97. https://doi.org/10.1126/science.aan3706 
|
| [70] |
Buchbinder EI, Desai A (2016) CTLA-4 and PD-1 pathways: Similarities, differences, and implications of their inhibition. Amn J Clin Oncolo 39: 98-106. https://doi.org/10.1097/COC.0000000000000239 
|
| [71] |
Alsaab HO, Sau S, Alzhrani R, et al. (2017) PD-1 and PD-L1 checkpoint signaling inhibition for cancer immunotherapy: Mechanism, combinations, and clinical outcome. Front Pharmacol 8: 561. https://doi.org/10.3389/fphar.2017.00561 
|
| [72] |
Champiat S, Dercle L, Ammari S, et al. (2017) Hyperprogressive disease is a new pattern of progression in cancer patients treated by anti-PD-1/PD-L1. Clin Cancer Res 23: 1920-1928. https://doi.org/10.1158/1078-0432.CCR-16-1741 
|
| [73] |
Wang Y, Zhou S, Yang F, et al. (2019) Treatment-related adverse events of PD-1 and PD-L1 inhibitors in clinical trials: A systematic review and meta-analysis. JAMA Oncol 5: 1008. https://doi.org/10.1001/jamaoncol.2019.0393 
|
| [74] |
Yi M, Zheng X, Niu M, et al. (2022) Combination strategies with PD-1/PD-L1 blockade: Current advances and future directions. Mol Cancer 21: 28. https://doi.org/10.1186/s12943-021-01489-2 
|
| [75] |
Akinleye A, Rasool Z (2019) Immune checkpoint inhibitors of PD-L1 as cancer therapeutics. J Hematol Oncol 12: 92. https://doi.org/10.1186/s13045-019-0779-5 
|
| [76] |
Debela DT, Muzazu SG, Heraro KD, et al. (2021) New approaches and procedures for cancer treatment: Current perspectives. SAGE Open Med 9. https://doi.org/10.1177/20503121211034366 
|
| [77] |
Goldhirsch A, Winer EP, Coates AS, et al. (2013) Personalizing the treatment of women with early breast cancer: Highlights of the St gallen international expert consensus on the primary therapy of early breast cancer 2013. Ann Oncol 24: 2206-2223. https://doi.org/10.1093/annonc/mdt303 
|
| [78] |
Shi J, Kantoff PW, Wooster R, et al. (2017) Cancer nanomedicine: Progress, challenges and opportunities. Nat Rev Cancer 17: 20-37. https://doi.org/10.1038/nrc.2016.108 
|
| [79] |
Bukowski K, Kciuk M, Kontek R (2020) Mechanisms of multidrug resistance in cancer chemotherapy. Int J Mol Sci 21: 3233. https://doi.org/10.3390/ijms21093233 
|
| [80] |
Jing X, Yang F, Shao C, et al. (2019) Role of hypoxia in cancer therapy by regulating the tumor microenvironment. Mol Cancer 18: 157. https://doi.org/10.1186/s12943-019-1089-9 
|
| [81] |
Yao Y, Zhou Y, Liu L, et al. (2020) Nanoparticle-based drug delivery in cancer therapy and its role in overcoming drug resistance. Front Mol Biosci 7: 193. https://doi.org/10.3389/fmolb.2020.00193 
|
| [82] |
Sun T, Zhang YS, Pang B, et al. (2014) Engineered nanoparticles for drug delivery in cancer therapy. Angew Chem Int Ed 53: 12320-12364. https://doi.org/10.1002/anie.201403036 
|
| [83] |
Anjum S, Ishaque S, Fatima H, et al. (2021) Emerging applications of nanotechnology in healthcare systems: Grand challenges and perspectives. Pharmaceuticals 14: 707. https://doi.org/10.3390/ph14080707 
|
| [84] |
Moore ZS, Seward JF, Lane JM (2006) Smallpox. Lancet 367: 425-435. https://doi.org/10.1016/S0140-6736(06)68143-9 
|
| [85] |
Hoover HC, Surdyke MG, Dangel RB, et al. (1985) Prospectively randomized trial of adjuvant active-specific immunotherapy for human colorectal cancer. Cancer 55: 1236-1243. https://doi.org/10.1002/1097-0142(19850315)55:6<1236::aid-cncr2820550616>3.0.co;2-# 
|
| [86] |
Miao L, Zhang Y, Huang L (2021) mRNA vaccine for cancer immunotherapy. Mol Cancer 20: 41. https://doi.org/10.1186/s12943-021-01335-5 
|
| [87] |
Saxena M, van der Burg SH, Melief CJM, et al. (2021) Therapeutic cancer vaccines. Nat Rev Cancer 21: 360-378. https://doi.org/10.1038/s41568-021-00346-0 
|
| [88] |
Le DT, Pardoll DM, Jaffee EM (2010) Cellular vaccine approaches. Cancer J 16: 304. https://doi.org/10.1097/PPO.0b013e3181eb33d7 
|
| [89] |
Farhood B, Najafi M, Mortezaee K (2019) CD8+ cytotoxic T lymphocytes in cancer immunotherapy: A review. J Cell Physiol 234: 8509-8521. https://doi.org/10.1002/jcp.27782 
|
| [90] |
Fang RH, Hu CMJ, Luk BT, et al. (2014) Cancer cell membrane-coated nanoparticles for anticancer vaccination and drug delivery. Nano Lett 14: 2181-2188. https://doi.org/10.1021/nl500618u 
|
| [91] |
Yada K, Nogami K, Ogiwara K, et al. (2013) Activated prothrombin complex concentrate (APCC)-mediated activation of factor (F)VIII in mixtures of FVIII and APCC enhances hemostatic effectiveness. J Thromb Haemostasis 11: 902-910. https://doi.org/10.1111/jth.12197 
|
| [92] |
Kennedy R, Celis E (2008) Multiple roles for CD4+ T cells in anti-tumor immune responses. Immunol Rev 222: 129-144. https://doi.org/10.1111/j.1600-065X.2008.00616.x 
|
| [93] | Rossella S, Trovato M, Manco R, et al. (2021) Exploiting viral sensing mediated by Toll-like receptors to design innovative vaccines. NPJ Vaccines 6. https://doi.org/10.1038/s41541-021-00391-8 |
| [94] |
Asiry S, Kim G, Filippou PS, et al. (2021) The cancer cell dissemination machinery as an immunosuppressive niche: A new obstacle towards the era of cancer immunotherapy. Front Immunol 12. https://doi.org/10.3389/fimmu.2021.654877 
|
| [95] |
Ott PA, Hodi FS, Robert C (2013) CTLA-4 and PD-1/PD-L1 blockade: New immunotherapeutic modalities with durable clinical benefit in melanoma patients. Clin Cancer Res 19: 5300-5309. https://doi.org/10.1158/1078-0432.CCR-13-0143 
|
| [96] |
Hargrave A, Mustafa AS, Hanif A, et al. (2023) Recent advances in cancer immunotherapy with a focus on FDA-approved vaccines and neoantigen-based vaccines. Vaccines 11: 1633. https://doi.org/10.3390/vaccines11111633 
|
| [97] |
Osipov A, Murphy A, Zheng L (2019) Chapter two-from immune checkpoints to vaccines: The past, present and future of cancer immunotherapy. Advances in Cancer Research . New York: Academic Press 63-144. https://doi.org/10.1016/bs.acr.2019.03.002 
|
| [98] |
Danila DC, Pantel K, Fleisher M, et al. (2011) Circulating tumors cells as biomarkers: Progress toward biomarker qualification. Cancer J 17: 438. https://doi.org/10.1097/PPO.0b013e31823e69ac 
|
| [99] |
Rizzo A, Ricci AD, Brandi G (2021) PD-L1, TMB, MSI, and other predictors of response to immune checkpoint inhibitors in biliary tract cancer. Cancers 13: 558. https://doi.org/10.3390/cancers13030558 
|
| [100] |
Chae YK, Pan A, Davis AA, et al. (2016) Biomarkers for PD-1/PD-L1 blockade therapy in non–small-cell lung cancer: Is PD-L1 expression a good marker for patient selection?. Clin Lung Cancer 17: 350-361. https://doi.org/10.1016/j.cllc.2016.03.011 
|
| [101] |
Li K, Tian H (2019) Development of small-molecule immune checkpoint inhibitors of PD-1/PD-L1 as a new therapeutic strategy for tumour immunotherapy. J Drug Targeting 27: 244-256. https://doi.org/10.1080/1061186X.2018.1440400 
|
| [102] |
Long J, Lin J, Wang A, et al. (2017) PD-1/PD-L blockade in gastrointestinal cancers: Lessons learned and the road toward precision immunotherapy. J Hematol Oncol 10: 146. https://doi.org/10.1186/s13045-017-0511-2 
|
| [103] |
Mas-Ponte D, McCullough M, Supek F (2022) Spectrum of DNA mismatch repair failures viewed through the lens of cancer genomics and implications for therapy. Clin Sc 136: 383-404. https://doi.org/10.1042/CS20210682 
|
| [104] |
Mardis ER (2019) Neoantigens and genome instability: Impact on immunogenomic phenotypes and immunotherapy response. Genome Med 11: 71. https://doi.org/10.1186/s13073-019-0684-0 
|
| [105] |
Shimozaki K, Hayashi H, Tanishima S, et al. (2021) Concordance analysis of microsatellite instability status between polymerase chain reaction based testing and next generation sequencing for solid tumors. Sci Rep 11: 20003. https://doi.org/10.1038/s41598-021-99364-z 
|
| [106] |
Gomez-Martín C, Lopez-Rios F, Aparicio J, et al. (2014) A critical review of HER2-positive gastric cancer evaluation and treatment: From trastuzumab, and beyond. Cancer Lett 351: 30-40. https://doi.org/10.1016/j.canlet.2014.05.019 
|
| [107] |
Duffy MJ, Crown J (2019) Biomarkers for predicting response to immunotherapy with immune checkpoint inhibitors in cancer patients. Clin Chem 65: 1228-1238. https://doi.org/10.1373/clinchem.2019.303644 
|
| [108] |
Riboldi M, Orecchia R, Baroni G (2012) Real-time tumour tracking in particle therapy: Technological developments and future perspectives. Lancet Oncol 13: e383-e391. https://doi.org/10.1016/S1470-2045(12)70243-7 
|
| [109] |
Danesi R, Fogli S, Indraccolo S, et al. (2021) Druggable targets meet oncogenic drivers: Opportunities and limitations of target-based classification of tumors and the role of Molecular Tumor Boards. ESMO Open 6: 100040. https://doi.org/10.1016/j.esmoop.2020.100040 
|
| [110] |
Sanchez A, Bocklage T (2019) Precision cytopathology: Expanding opportunities for biomarker testing in cytopathology. J Am Soc Cytopathol 8: 95-115. https://doi.org/10.1016/j.jasc.2018.12.003 
|
| [111] |
Łukaszewicz-Zając M, Pączek S, Muszyński P, et al. (2019) Comparison between clinical significance of serum CXCL-8 and classical tumor markers in oesophageal cancer (OC) patients. Clin Exp Med 19: 191-199. https://doi.org/10.1007/s10238-019-00548-9 
|
| [112] |
Roessler S, Jia HL, Budhu A, et al. (2010) A unique metastasis gene signature enables prediction of tumor relapse in early-stage hepatocellular carcinoma patients. Cancer Res 70: 10202-10212. https://doi.org/10.1158/0008-5472.CAN-10-2607 
|
| [113] |
Sun Z, Zhang N (2014) Clinical evaluation of CEA, CA19-9, CA72-4 and CA125 in gastric cancer patients with neoadjuvant chemotherapy. World J Surg Onc 12: 397. https://doi.org/10.1186/1477-7819-12-397 
|
| [114] |
Lakemeyer L, Sander S, Wittau M, et al. (2021) Diagnostic and prognostic value of CEA and CA19-9 in colorectal cancer. Diseases 9: 21. https://doi.org/10.3390/diseases9010021 
|
| [115] |
Jing JX, Wang Y, Xu XQ, et al. (2015) Tumor markers for diagnosis, monitoring of recurrence and prognosis in patients with upper gastrointestinal tract cancer. Asian Pac J Cancer Prev 15: 10267-10272. https://doi.org/10.7314/APJCP.2014.15.23.10267 
|
| [116] |
Galle PR, Foerster F, Kudo M, et al. (2019) Biology and significance of alpha-fetoprotein in hepatocellular carcinoma. Liver Int 39: 2214-2229. https://doi.org/10.1111/liv.14223 
|
| [117] |
He R, Yang Q, Dong X, et al. (2017) Clinicopathologic and prognostic characteristics of alpha-fetoprotein–producing gastric cancer. Oncotarget 8: 23817. https://doi.org/10.18632/oncotarget.15909 
|
| [118] |
Madhavan D, Peng C, Wallwiener M, et al. (2016) Circulating miRNAs with prognostic value in metastatic breast cancer and for early detection of metastasis. Carcinogenesis 37: 461-470. https://doi.org/10.1093/carcin/bgw008 
|
| [119] |
Hamam R, Hamam D, Alsaleh KA, et al. (2017) Circulating microRNAs in breast cancer: Novel diagnostic and prognostic biomarkers. Cell Death Dis 8: e3045-e3045. https://doi.org/10.1038/cddis.2017.440 
|
| [120] |
Li RY, Liang ZY (2020) Circulating tumor DNA in lung cancer: real-time monitoring of disease evolution and treatment response. Chin Med J 133: 2476-2485. https://doi.org/10.1097/CM9.0000000000001097 
|
| [121] |
Zhou H, Shen W, Zou H, et al. (2020) Circulating exosomal long non-coding RNA H19 as a potential novel diagnostic and prognostic biomarker for gastric cancer. J Int Med Res 48: 0300060520934297. https://doi.org/10.1177/0300060520934297 
|
| [122] |
Jiang L, Gu Y, Du Y, et al. (2019) Exosomes: Diagnostic biomarkers and therapeutic delivery vehicles for cancer. Mol Pharmaceutics 16: 3333-3349. https://doi.org/10.1021/acs.molpharmaceut.9b00409 
|
| [123] |
Huyghe N, Benidovskaya E, Stevens P, et al. (2022) Biomarkers of response and resistance to immunotherapy in microsatellite stable colorectal cancer: Toward a new personalized medicine. Cancers 14: 2241. https://doi.org/10.3390/cancers14092241 
|
| [124] |
Colle R, Cohen R, Cochereau D, et al. (2017) Immunotherapy and patients treated for cancer with microsatellite instability. Bull Cancer 104: 42-51. https://doi.org/10.1016/j.bulcan.2016.11.006 
|
| [125] |
He CY, Qiu MZ, Yang XH, et al. (2020) Classification of gastric cancer by EBV status combined with molecular profiling predicts patient prognosis. Clin Transl Med 10: 353-362. https://doi.org/10.1002/ctm2.32 
|
| [126] |
Shen H, Zhong M, Wang W, et al. (2017) EBV infection and MSI status significantly influence the clinical outcomes of gastric cancer patients. Clin Chim Acta 471: 216-221. https://doi.org/10.1016/j.cca.2017.06.006 
|
| [127] |
Yuan L, Xu ZY, Ruan SM, et al. (2020) Long non-coding RNAs towards precision medicine in gastric cancer: Early diagnosis, treatment, and drug resistance. Mol Cancer 19: 96. https://doi.org/10.1186/s12943-020-01219-0 
|
| [128] |
Cao F, Hu Y, Chen Z, et al. (2021) Circulating long noncoding RNAs as potential biomarkers for stomach cancer: A systematic review and meta-analysis. World J Surg Onc 19: 89. https://doi.org/10.1186/s12957-021-02194-6 
|
| [129] |
Li J, Wu Z, Lin R (2024) Impact of Helicobacter pylori on immunotherapy in gastric cancer. J Immunother Cancer 12: e010354. https://doi.org/10.1136/jitc-2024-010354 
|
| [130] | Zhong X, Zheng H, Zhao S, et al. (2024) Effects and mechanisms of Helicobacter pylori on cancers development and immunotherapy. Front Immunol 15. https://doi.org/10.3389/fimmu.2024.1469096 |
| [131] |
Wang Y, Shatila M, Sperling G, et al. (2024) Helicobacter pylori infection and response of gastric cancer to immunotherapy. J Clin Oncol 42: 277-277. https://doi.org/10.1200/JCO.2024.42.3_suppl.277 
|
| [132] |
Magahis PT, Maron SB, Cowzer D, et al. (2023) Impact of Helicobacter pylori infection status on outcomes among patients with advanced gastric cancer treated with immune checkpoint inhibitors. J Immunother Cancer 11: e007699. https://doi.org/10.1136/jitc-2023-007699 
|
| [133] |
Hegde PS, Chen DS (2020) Top 10 challenges in cancer immunotherapy. Immunity 52: 17-35. https://doi.org/10.1016/j.immuni.2019.12.011 
|
| [134] |
Chen K, Shuen TWH, Chow PKH (2024) The association between tumour heterogeneity and immune evasion mechanisms in hepatocellular carcinoma and its clinical implications. Br J Cancer 131: 420. https://doi.org/10.1038/s41416-024-02684-w 
|
| [135] |
Chowell D, Morris LGT, Grigg CM, et al. (2018) Patient HLA class I genotype influences cancer response to checkpoint blockade immunotherapy. Science 359: 582-587. https://doi.org/10.1126/science.aao4572 
|
| [136] |
Schneider BJ, Naidoo J, Santomasso BD, et al. (2021) Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: ASCO guideline update. J Clin Oncol 39: 4073-4126. https://doi.org/10.1200/JCO.21.01440 
|
| [137] |
Gopalakrishnan V, Spencer CN, Nezi L, et al. (2018) Gut microbiome modulates response to anti–PD-1 immunotherapy in melanoma patients. Science 359: 97-103. https://doi.org/10.1126/science.aan4236 
|
| [138] |
Pagliuca S, Gurnari C, Rubio MT, et al. (2022) Individual HLA heterogeneity and its implications for cellular immune evasion in cancer and beyond. Front Immunol 13. https://doi.org/10.3389/fimmu.2022.944872 
|
| [139] |
Mitchell AL, Gandhi A, Scott-Coombes D, et al. (2016) Management of thyroid cancer: United kingdom national multidisciplinary guidelines. J Laryngol Otol 130: S150-S160. https://doi.org/10.1017/S0022215116000578 
|
| [140] |
Dhatchinamoorthy K, Colbert JD, Rock KL (2021) Cancer Immune evasion through loss of MHC class I antigen presentation. Front Immunol 12: 636568. https://doi.org/10.3389/fimmu.2021.636568 
|
| [141] |
Postow MA, Sidlow R, Hellmann MD (2018) Immune-related adverse events associated with immune checkpoint blockade. N Engl J Med 378: 158-168. https://doi.org/10.1056/NEJMra1703481 
|
| [142] |
Brahmer JR, Lacchetti C, Schneider BJ, et al. (2018) Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American society of clinical oncology clinical practice guideline. J Clin Oncol 36: 1714-1768. https://doi.org/10.1200/JCO.2017.77.6385 
|