1.
Introduction
Arteriovenous malformations (AVMs) are fast-flow vascular malformations comprised of complex networks of primitive vessels directly connecting feeding arteries to draining veins and lacking a normal capillary network [1]; the area containing the abnormal vasculature and shunting is referred to as the nidus.
The behavior of extracranial AVMs is locally aggressive. As AVMs progress, they destroy normal tissues, eventually leading to complications, such as severe disfigurement, uncontrollable bleeding, ulceration, pain, and cardiac volume overload. The only currently available curative AVM treatment is complete removal or ablation of the nidus, with complete radical resection representing the recommended surgical treatment [2]–[4]. The radical resection of a AVM mass can be very difficult, resulting in disfigurement and functional damage, particularly in the head and neck region, where AVMs commonly occur. Completely improved outcome was observed in only 35–60% of patients treated at the hospital [5]. In addition, there is a high chance of recurrence or progression of AVMs, even after treatment: Recurrence rates of over 80% after embolization and surgical resection have been reported [6], although recent advances and improved multidisciplinary care approaches may result in future improvements. Recurrent mechanisms were explained by some hypotheses such as hypoxia, trauma, inflammation, even embolic and surgical treatments. The aim of the present study was to identify correlations between patterns of extracranial head and neck AVM presentations and the frequency of recurrence.
2.
Materials and methods
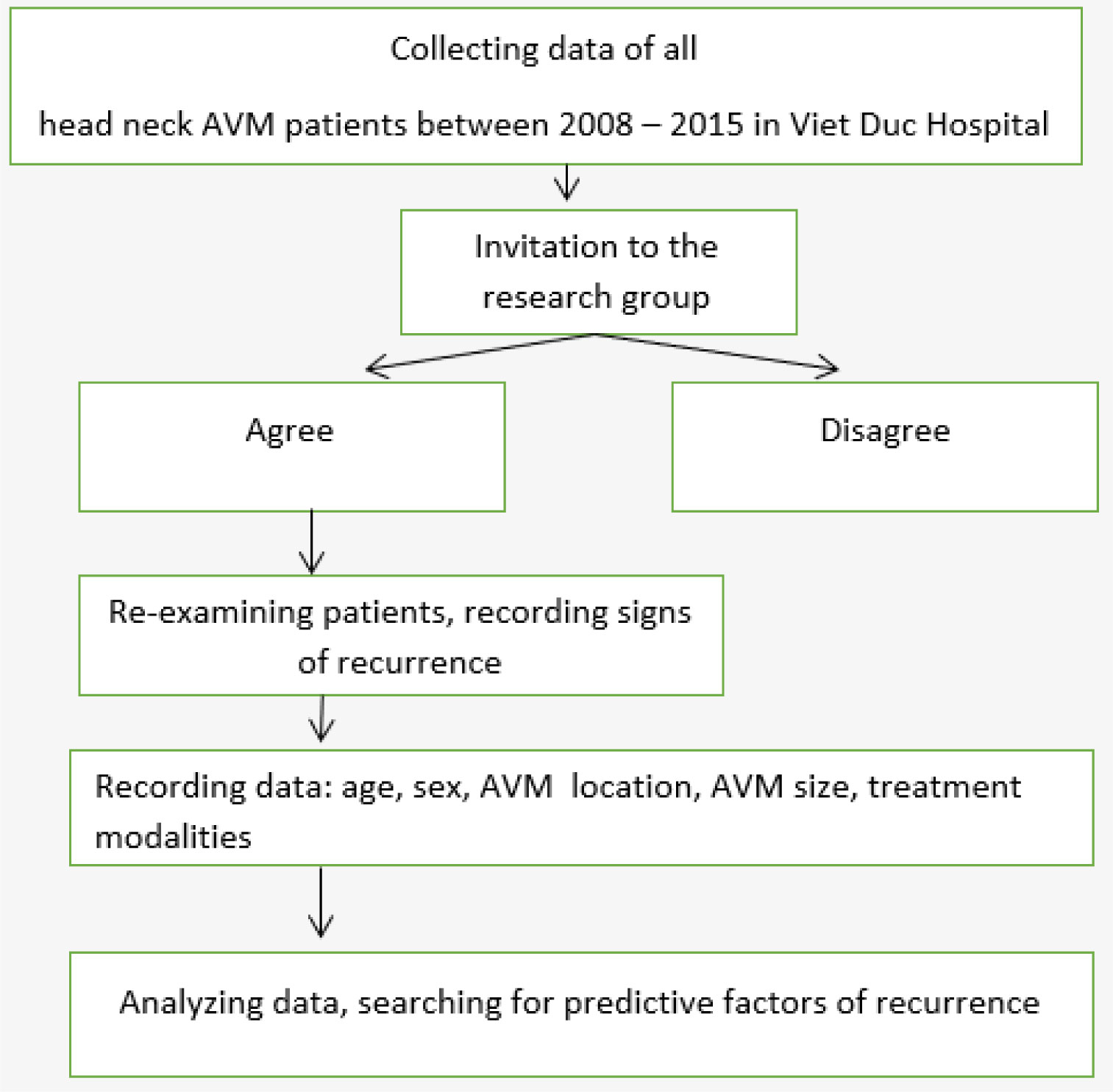
This retrospective study was approved by the Ethics Committee of Hanoi Medical University (20NCS17/HMU IRB). Informed consent from patients was obtained or waived. Flow chart of this study was introduced in Figure 1.
We reviewed all patients with head and neck AVMs who were treated by the Department of Maxillofacial and Plastic Surgery, Viet Duc Hospital, between January 2008 and December 2015. Using chart review, we recorded the location, clinical symptoms, clinical stage according to the Schöbinger staging system (Table 1), and treatment modalities applied to each AVM case.
To determine clinical or imaging indications of recurrence after treatment, we reviewed all documents associated with all follow-up clinical appointments and reports associated with any post-treatment Doppler ultrasound, magnetic resonance imaging (MRI), or digital subtraction angiography (DSA) studies.
We considered the reappearance of a mass, pulsation, or both, to represent a clinical sign of recurrence. The reappearance of a mass, flow voids, or both (on MRI), or any indication of arteriovenous shunting (DSA or Doppler ultrasound) were recorded as imaging signs of recurrence.
Age, sex, AVM location, AVM size, and treatment modalities were recorded to determine whether any of these variables were predictive factors for AVM recurrence.
Data were analyzed using SPSS v.20.0. Data are presented as the mean with 95 percent confidence intervals or standard deviation. Proportions were compared using Chi-square analysis and Fisher's exact test. Two-tailed values of p < 0.05 were considered significant.
3.
Results
A total of 55 patients met the selection criteria and were enrolled in the study. The mean age at the time of treatment was 29.9 ± 12.73 years (range: 3–67 years). The male to female ratio was 2 to 1. The Schöbinger stage was II in 38 patients and III in 17 patients. No patients were classified as Schöbinger stages I or IV (Table 2).
All patients were treated using a combination of embolization and surgical resection (Figures 2 and 3).
N-butyl-2-cyanoacrylate (NBCA) was typically used to embolize the nidus. In complicated cases, other agents were also used, including polyvinyl alcohol (PVA), Amplatzer, coils, and gelatin sponge plugs. Superselective intra-arterial embolization (SIAE) was performed in 30 patients (54.5%); direct puncture into the nidus was performed in 25 patients, either alone or combined with SIAE.
Total resection of the nidus was performed in 50 patients, whereas the remaining 5 patients presented with large and diffuse AVMs that could not be totally removed. To cover defects following resection, direct closure was applied in 41 patients. Skin grafts were required in 5 patients, local flaps were used in 11 patients, and a distal flap was used in 1 patient. No patients were treated using free flaps.
AVM recurrence following treatment was documented in 25.5% of patients during an average follow-up time of 3.5 ± 2.02 years.
Multivariate analysis showed that age (p = 0.703) and AVM location (p = 0.32) were not correlated with recurrence. By contrast, sex (p = 0.045), Schöbinger stage (p = 0.005), AVM size (p = 0.026), and treatment modality (p = 0.001) were independent predictors of recurrence. Patients managed by total resection had the lowest recurrence rate compared with patients who were managed by subtotal resection.
4.
Discussion
Current management of AVMs generally involves close observation, surgical excision, intravascular embolization, or some combination of these treatment modalities. Extracranial head and neck AVM are a locally aggressive lesion located in a complex anatomical region, associated with a high recurrence rate after intervention [2],[8]. The pathogenesis of head and neck AVMs remains unclear [1],[9]–[12].
In a series of 272 patients with head and neck AVMs reported by Liu et al., which underscored the difficulty of achieving a long-term cure for peripheral AVM, the reported recurrence rates after surgical resection were 81% for head AVMs and 98% for neck AVMs after embolization [5]. In a series of 81 patients, Kohout et al. reported an overall cure rate of 60% [5]. In our series, the recurrence rate was 25.5%, which may be associated with the shorter follow-up for our study, which averaged only 3.5 years compared with 4.6 years reported by Liu et al. study and 12.7 years reported by Kohout et al. According to Fernández-Alvarez and Richter, recurrence can occur as long as 10 years after treatment [13],[14].
Koshima, Pekkola, Kohout and some other authors have suggested that recurrence mechanisms include a proangiogenic environment involving hypoxia, trauma, and inflammation, embolic and surgical treatments which can support the continued development of any remaining nidus remnant, leading to recurrence, often with a complex architecture and extensive vascular recruitment [2],[5],[12],[15].
Our results suggest that early and total nidus resection can reduce the risk of recurrence. Richter and Suen also supposed that vigilant observation, early treatment, and radical therapy are necessary for AVMs of the head and neck [14]. However, total resection is not always easy or possible, particularly for large and diffuse lesions in the head and neck region [1],[16],[17]. We postulate that the use of a pedicled or free flap to cover the tissue defect after resection may minimize AVM recurrence by reducing tissue hypoxia [15].
In our series, the 55 patients with head and neck AVMs were categorized as Schöbinger stages II or III, and 8 of 18 female patients experienced recurrence (44.4%), which was a significantly higher rate than observed in male patients (16.2%; p = 0.024). This finding differs from that reported by Liu et al., for which the recurrence rate was not correlated with sex [6]. In our study group, 2 of the patients who experienced recurrence were pregnant. Some authors [9],[18] believe that pregnancy or puberty may increase the risk of progression because hormonal changes associated with these periods may stimulate AVMs by promoting angiogenesis. However, in quiescent lesions (stage I), Liu et al. did not find progression in pregnant women [6]. The rate of recurrence was 30% (n = 3) among the younger group and 24.4% (n = 11) among the adult group, which was not significantly different.
Of the 14 patients who had auricular AVMs, 8 patients experienced recurrence, which may be due to the surgical treatment, as some patients (n = 10; 71.4%) were not prepared to undergo auricular resection and preferentially chose embolization and partial nidus resection. However, this recurrence rate was not significantly higher than that for other AVM locations (p = 0.32). In the series of 41 patients with auricular AVMs (Wu et al.), 20 patients (48.78%) had recurrence after mean time of 5.19 years follow-up. The high rate of recurrence in auricular region might be explained by patients' desire of auricular conservation at the lower-staged and the recurrent tendency after treatment at higher-staged AVMs. According to Lu et al., higher-staged AVMs exhibit increased expression of endothelial progenitor cells and factors that stimulate their recruitment [18].
Although the pathology underlying AVM development remains unclear, Lu and Colletti postulated that pharmacotherapy may be able to control the re-expansion and recurrence through the inhibition of vascular endothelial growth factor and angiopoietin 2, which have been detected in AVM tissue [18],[19].
5.
Conclusions
The management of AVMs that occur in the head and neck region is challenging and requires a multidisciplinary approach. The rate of recurrence after treatment is high, particularly for diffuse AVMs. Sex, stage, and AVM size, in addition to the treatment modality, were predictive factors for recurrence. Early treatment allowing for total AVM resection is the optimal approach for preventing recurrence.








 DownLoad:
DownLoad:





